Picture yourself standing on the edge of a primordial world, where the sky crackles with electrical fury and every bolt carries the potential to birth life itself. You’re witnessing something extraordinary happening beneath your feet that would fundamentally alter the course of our planet’s destiny. The story of how lightning first transformed Earth isn’t just about spectacular displays of nature’s power – it’s about the most profound chemical revolution our planet has ever experienced.
The violent dance between sky and ground that began roughly 4.6 billion years ago didn’t just light up ancient skies. It sparked an intricate chemical orchestra that would eventually compose the symphony of life. Let’s dive into this electrifying journey through time.
The Primordial Stage: Earth’s Lightning Laboratory

You need to understand that early Earth was nothing like the world you know today. About 4.6 billion years ago, Earth resembled the set of a summer sci-fi blockbuster. The planet’s surface was a harsh and barren landscape, recovering from hellish asteroid strikes, teeming with volcanic eruptions, and lacking enough nutrients to sustain even the simplest forms of life. The atmosphere was composed predominantly of inert gases like nitrogen and carbon dioxide, meaning they did not easily engage in chemical reactions necessary to form the complex organic molecules that are the building blocks of life.
The atmospheric composition of early Earth (the first billion years) was drastically different from its current state. Initially, hydrogen and helium compounds dominated the atmosphere. However, given the relatively small size of these elements and the warmer temperature of Earth compared to other planets at the time, most of these lighter compounds escaped, leaving behind an atmosphere composed mainly of methane, nitrogen, oxygen and ammonia with small concentrations of hydrogen compounds and other gases. This created the perfect conditions for lightning to begin its transformative work.
When the First Sparks Flew: Lightning’s Early Appearances

Lightning was more prevalent on early Earth because there was more carbon dioxide in the atmosphere. Carbon dioxide contributes to the global temperature, and a higher global temperature causes more frequent and intense thunderstorms. You can imagine the raw power coursing through these ancient storms, far exceeding anything witnessed today.
Today, about 560 million lightning bolts flash over the planet a year; 4 billion years ago, when Earth’s atmosphere was significantly richer in the greenhouse gas CO2 (and therefore hotter and more prone to storms), it’s likely that anywhere from 1 billion to 5 billion bolts flashed each year. This means you’re looking at a planet under constant electrical bombardment, creating perfect conditions for widespread chemical transformation.
The Chemical Miracle: How Lightning Unlocked Life’s Building Blocks

The scientists discovered that their simulated lightning strikes could transform stable gases like carbon dioxide and nitrogen into highly reactive compounds. They found that carbon dioxide could be reduced to carbon monoxide and formic acid, while nitrogen could be converted into nitrate, nitrite, and ammonium ions. This represents one of the most remarkable chemical transformations in planetary history.
In the study, published in Proceedings of the National Academy of Sciences, a team of Harvard scientists identified lightning-induced plasma electrochemistry as a potential source of reactive carbon and nitrogen compounds necessary for the emergence and survival of early life. The key insight here is that lightning didn’t just create random chemical chaos – it systematically generated the precise molecular ingredients needed for biological processes.
The Miller-Urey Revolution: Laboratory Lightning Reveals Life’s Secrets

Miller and Urey injected ammonia, methane and water vapor into an enclosed glass container to simulate what were then believed to be the conditions of Earth’s early atmosphere. Then they passed electrical sparks through the container to simulate lightning. Amino acids, the building blocks of proteins, soon formed. Miller and Urey realized that this process could have paved the way for the molecules needed to produce life.
So in 1953 a young American chemist named Stanley Miller filled a flask halfway with water, topped it with methane, ammonia and hydrogen to mimic the planet’s early atmosphere, and then flung a miniature lightning bolt into that fertile soup. In this landmark experiment, Miller, working with guidance from his research adviser Harold Urey, produced several amino acids out of inorganic molecules. This groundbreaking work proved that you could literally spark life from lifeless matter.
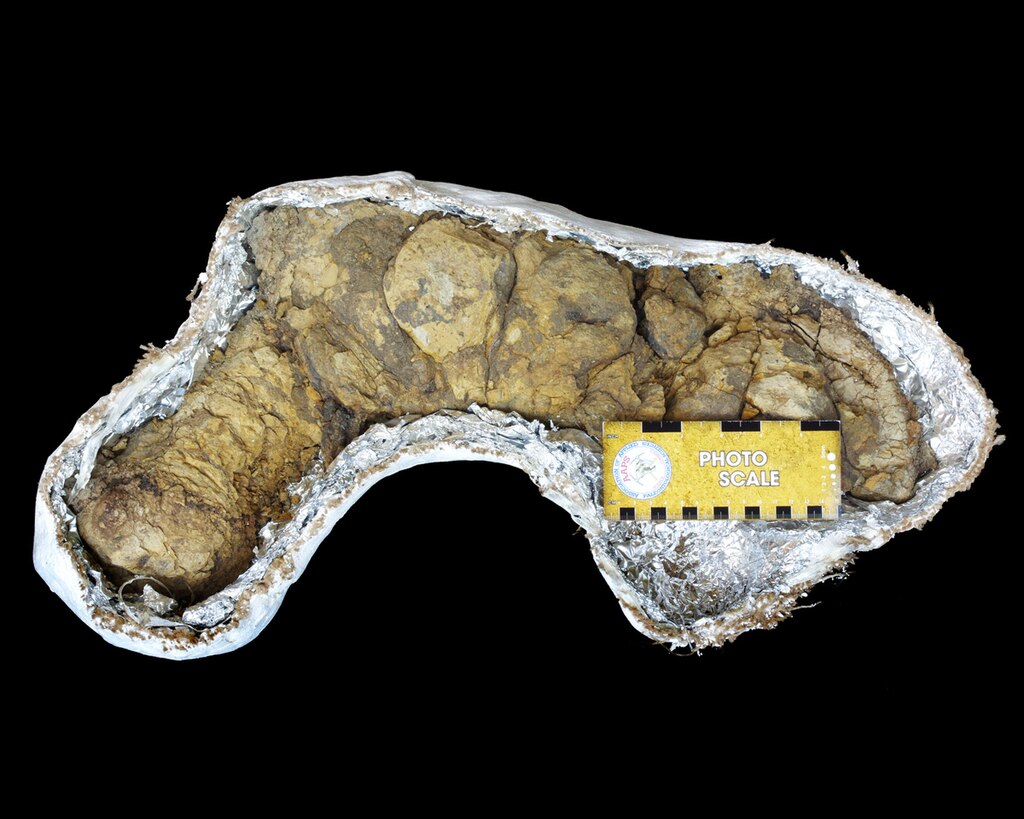
Fulgurites: Nature’s Glass Furnaces That Changed Everything

Schreibersite is also found in glass materials called fulgurites, which are formed when lighting hits Earth. When fulgurite forms, it incorporates phosphorus from terrestrial rocks. These extraordinary formations became nature’s chemical factories, transforming ordinary rock into life-enabling substances.
When a bolt strikes the soil it burns a molten, hollow tube called a fulgurite that Pasek says looks like a “mini volcano with glass splattered around it.” These fulgurites, which range from a few grams up to tens of kilograms, are not easy to find, and meteorite dealers often pay nomads in the Sahara Desert to collect them during their travels. Each one represents a snapshot of lightning’s transformative power frozen in glass.
The Phosphorus Problem: Lightning’s Solution to Life’s Bottleneck

Author Isaac Asimov once called phosphorus “life’s bottleneck,” because it makes up 1 percent of an organism but is only present in 0.1 percent of minerals on Earth. This scarcity created a fundamental challenge for early life that lightning uniquely solved.
One of the key ingredients needed for life as we know it is phosphorus – and the multitude of lightning strikes that happened when Earth was young 4.6 billion years ago may have unlocked the necessary amount of phosphorus to create the foundation for life. Phosphorus is needed in the molecules that form basic cell structures and cell membranes and even makes up the phosphate backbone of DNA and RNA. But this element was elusive on early Earth, trapped inside minerals. Lightning became the key that unlocked this biological treasure chest.
Volcanic Lightning: The Ultimate Chemical Reactor

It has been argued that volcano-induced lightning in the early stages of Earth’s existence, because the volcanic plume was composed of additional “reducing gases”, was more effective at stimulating the oxidation of organic material to accelerate the production of life. Since this process occurs fairly close to ground level, it has been suggested that volcanic lightning contributed to the generation of life to a greater extent than lightning produced within clouds that would lower positive or negative charge from a cloud to the ground.
Here I present a hypothesis that lightning associated with volcanic island eruptions created focal points for the generation of prebiotic ingredients and ultimately the origin of life. Lightning is often generated within ash clouds during volcanic eruptions, having been reported at nearly 400 eruptions of 152 volcanoes. These volcanic lightning events created concentrated zones of chemical activity that may have been Earth’s first biological laboratories.
The Global Lightning Network: Earth’s Chemical Distribution System

Here, we propose an alternative source for widespread phosphorus reduction, arguing that lightning strikes on early Earth potentially formed 10–1000 kg of phosphide and 100–10,000 kg of phosphite and hypophosphite annually. Therefore, lightning could have been a significant source of prebiotic, reactive phosphorus which would have been concentrated on landmasses in tropical regions.
Of those bolts, the team estimated that between 100 million and 1 billion bolts struck land each year (the rest discharged above the oceans). And, over a billion years, up to a quintillion (a 1 followed by 18 zeros) lightning strikes may have hit our young planet, each one releasing a bit of usable phosphorus. The team calculated that, between 4.5 billion and 3.5 billion years ago, lightning strikes alone could have given Earth anywhere from 250 to 25,000 pounds of phosphorus (110 to 11,000 kilograms) per year. This represents one of the most efficient chemical distribution networks our planet has ever known.
Beyond Earth: Lightning’s Universal Potential for Life

The study not only sheds light on the past but also has implications for the search for life on other planets. Processes the researchers described could potentially contribute to the emergence of life beyond Earth. “Lightning has been observed on Jupiter and Saturn; plasmas and plasma-induced chemistry can exist beyond our solar system,” Jiang said. “Moving forward, our setup is useful for mimicking environmental conditions of different planets, as well as exploring reaction pathways triggered by lightning and its analogs.”
Lightning strikes could likewise provide a continual source of prebiotic reactive phosphorus independent of meteorite flux on other Earth-like planets, potentially facilitating the emergence of terrestrial life indefinitely. This discovery transforms our understanding of where and how life might arise throughout the universe, suggesting that any world with lightning might possess the chemical foundation for biological evolution.
Conclusion

The story of lightning’s role in sparking life on Earth reveals one of nature’s most elegant solutions to an impossible problem. In a world where the essential ingredients for life were locked away in unreactive forms, electrical storms became the universal key that unlocked biological potential. From transforming stable atmospheric gases into reactive compounds to creating fulgurite furnaces that freed phosphorus from rock, lightning orchestrated a planetary-scale chemistry experiment that would culminate in the emergence of life itself.
Today, as you witness lightning illuminate modern skies, you’re seeing the same fundamental process that made your existence possible. Every bolt carries echoes of those ancient sparks that first taught lifeless matter how to become alive. What do you think about knowing that life itself might owe its existence to something as seemingly chaotic as a lightning storm?